
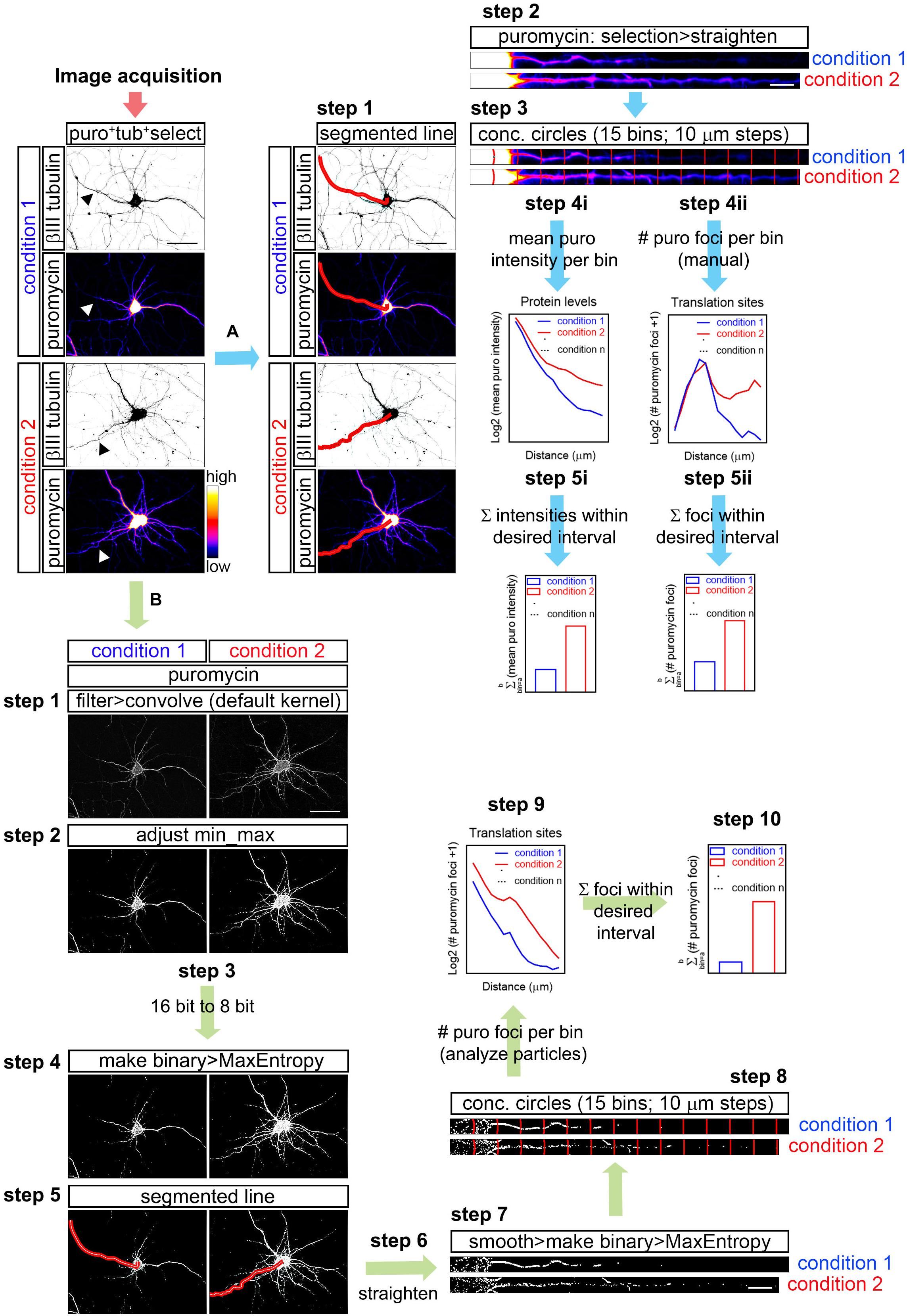
Overall, this manuscript discusses considerations for collecting quantifiable fluorescent images on a confocal microscope and provides explicit methods for quantitating IF data using FIJI-ImageJ. Matrix Biology, 2020 which lays out a workflow where IF data collected on a confocal microscope can be used to quantitate the relative levels of a molecule of interest by measuring mean fluorescent intensity across a region of interest, cell number, and the percentage of cells in a sample “positive” for staining with the fluorescent probe of interest. Here we describe the methods used to quantify the data presented in Shihan et al. While protein detection in tissue using immunofluorescent (IF) probes has traditionally been considered a qualitative technique, advances in probe stability and confocal imaging allow IF data to be easily quantitated, although reproducible quantitation of relative protein expression requires careful attention to appropriate controls, experiment design, and data collection. However, WB and ELISA have limited ability to meaningfully quantitate relative protein levels in tissues with complex cell composition, while tissue dissociation followed by FC is not feasible when tissue is limiting and/or cells difficult to isolate. Western blotting (WB), enzyme-linked immunosorbent assay (ELISA) and flow cytometry (FC) have long been used to assess and quantitate relative protein expression in cultured cells and tissue samples.


 0 kommentar(er)
0 kommentar(er)
